




TREATING MIGRAINE
MAY NEVER BE THE SAME 1
Today, with Precision Olfactory Delivery (POD®) technology, patients can have the proven effcacy of dihydroergotamine mesylate (DHE) on demand for rapid, sustained, consistent relief, even when taken late into a migraine attack.1,2,4-6
Download
resources:
Download resources:
The
evolution
of DHE for
at-home
use
Determining
the most
appropriate
acute
treatment

POD:
Beyond just
another
nasal spray

Treating
migraine
effectively
through
the nose

How do
you use
Trudhesa?
Just Be
DIRECT

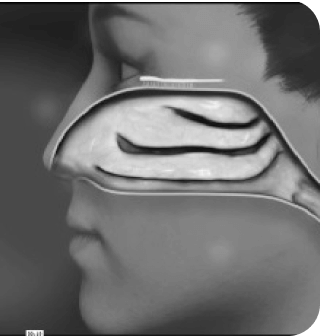
How does
POD
technology
work?

See POD
technology
in action

Trudhesa is an ergotamine derivative indicated for the acute treatment of migraine with or without aura in adults.
Trudhesa is not indicated for the preventive treatment of migraine or for the management of hemiplegic or basilar migraine.
Trudhesa is not recommended in patients with:
Trudhesa may cause:
Most common adverse reactions (incidence >1%) were rhinitis, nausea, altered sense of taste, application site reactions, dizziness, vomiting, somnolence, pharyngitis, and diarrhea.
Pregnancy : Available data from published literature indicate an increased risk of preterm delivery with Trudhesa use during pregnancy.
Lactation : Patients should not breastfeed during treatment with Trudhesa and for 3 days after the last dose.
Please see the Trudhesa Full Prescribing Information, including Boxed Warning and Medication Guide.
The risk information provided here is not comprehensive. The FDA-approved product labeling can be found at www.trudhesaHCP.com or 1-800-555-DRUG. You can also call 1-833-TRUDHESA (1-833-878-3437) for additional information.
References: 1. Trudhesa. Prescribing information. Impel Neuropharma; 2021. 2. Shrewsbury SB, Jeleva M, Satterly KH, Lickliter J, Hoekman J. STOP 101: a phase 1, randomized, open-label, comparative bioavailability study of INP104, dihydroergotamine mesylate (DHE) administered intranasally by a I123 Precision Olfactory Delivery (POD®) Device, in healthy adult subjects. Headache. 2019;59(3):394-409. 3. Smith TR, Aurora S, Hocevar-Trnka J, Shrewsbury SB. Acute treatment of migraine with INP104: exploratory e£cacy from the phase 3 STOP 301 study. Poster presented at: American Headache Society Virtual Annual Scientific Meeting, June 3-6, 2021. 4. Smith TR, Winner P, Azzzurora SK, Jeleva M, Hocevar-Trnka J, Shrewsbury SB. STOP 301: a phase 3, open-label study of safety, tolerability, and exploratory e£cacy of INP104, Precision Olfactory Delivery (POD®) of dihydroergotamine mesylate, over 24/52 weeks in acute treatment of migraine attacks in adult patients [published online ahead of print, 2021 Aug 7]. Headache. 2021;10.1111/head.14184. doi:10.1111/head.14184. 5. Silberstein SD, Shrewsbury SB, Hoekman J. Dihydroergotamine (DHE) - then and now: a narrative review. Headache. 2020;60(1):40-57. 6. Data on File. Impel Neuropharma. 2020.
.png)
.png)
IMPEL, POD, TRUDHESA DIRECT, and the Impel and Trudhesa Logos are trademarks of Impel NeuroPharma, Inc.
© 2022 Impel NeuroPharma, Inc. All Rights Reserved. US-TRU-2200054 03/2022